


Associate Professor
Publications
Group Members

SRF

SRF

JRF

JRF

JRF

Past lab members
Research
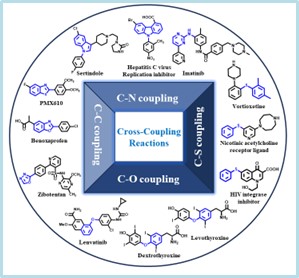
Novel catalytic strategies for cross-coupling reactions
Chemical framework of organic compounds mainly constituted through the C-C and C-X (X= N, S, O) bonds. Naturally, chemical methods that allow the C-C or C-X (X= N, S, O) bond formations are extremely important in organic synthesis. My research group mainly focuses on the development of novel catalytic strategies for the C-C and C-X (X= N, S, O) cross coupling reactions for the selective functionalization of a variety of heterocycles. We are particularly interested in C-H bond activation reactions which provides efficient and practical avenues for C-C cross coupling reactions. Our work in this area includes:
Palladium catalyzed direct C–H arylation of heteroarenes.
Over the past few decades, the field of C-H bond activation has evolved from a research concept to a powerful tool for the construction of C-C or C-heteroatom bonds. Indeed, recent years have witnessed a remarkable progress in the C-H bond activation strategies with promising application to pharmaceutical product syntheses, natural product syntheses and material sciences. While development of transition metal catalyzed direct functionalization of C-H bonds has continued to thrive, a low reactivity and selectivity of C-H bonds poses an inherent challenge. The key challenges in direct C-H arylation reactions are delineated in Figure 1.

Figure 1. Key challenges in C – H bond arylations reactions.
To tackle these challenges, we are exploring new catalytic strategies for efficient cleavage of latent C-H bonds of heteroarenes and subsequent functionalization. In particular, we are interested in developing new methods for the direct C-H arylation of heteroarenes. Synthetic procedures, that enable the selective arylation of heteroarene, are of particular interest to organic chemists since arylated heteroarenes derivatives forms a core structure of many biologically active compounds as well as valuable organic materials.
Cu catalyzed carbon-heteroatom (C-X) cross-coupling reactions.
Over the years, transition metal catalyzed C-C and C-heteroatom cross coupling reactions has received considerable attention since these reactions exhibit a wide application in both academy and industry. Most of these coupling reactions has relied heavily on the catalytic properties of p precious metal complexes as catalysts. The low-abundance, expensiveness and air-sensitive nature of precious metals limit their application on the larger-scale. Besides, low abundance of precious metals can definitely increase the carbon footprint during their extraction. Hence, catalytic strategies involving earth-abundant, more environmental-friendly, non-precious metals such as copper are in great demand. However, Cu catalysts suffered from certain shortcomings over other transition metal catalysts. The requirement of harsh reaction conditions (high temperature and strong bases), narrow substrate scope, high catalyst loading and moderate yields in copper catalyzed reactions confines their use in laboratory as well as in the industrial settings. To address these issues, we pursue improvement in the copper-catalyzed C-N cross coupling methods. Past few years, we have developed selective, efficient and scalable methods for the Cu catalyzed C-N and C-S cross coupling reactions. We use mechanism-based method development approach wherein we use various experimental and computational tools to understand the mechanism of reactions as well as role of catalyst. Such molecular-level understanding on the mechanism of copper-catalyzed reactions can be used for the design and develop new bidentated ligands for copper-catalysis. In general, aryl chlorides/bromides are found to be inactive coupling partners in the copper-catalyzed reactions because of the low reactivity of aryl chlorides/bromides in the oxidative addition step. In our laboratory, we are trying to develop new copper-catalysts which allow efficient involvement of aryl chlorides/bromides in the cross-coupling reactions.
UM-DAE Centre for Excellence in Basic Sciences
Nalanda Building, University of Mumbai, Vidyanagari, Mumbai 400098, India.
NEST/Outreach:+9186570 26481
NEST/Admissions:+9186570 26482
General Enquiries: info@cbs.ac.in
Admissions Queries:admissions@cbs.ac.in
Web: https://cbs.ac.in